Mole Fraction Tutorial :
Formula :
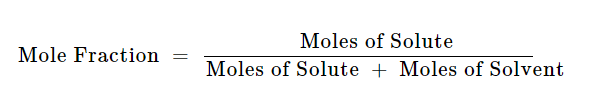
Example-1: 0.100 mole of NaCl is dissolved into 100.0 grams of pure H2O. What is the mole fraction of NaCl?
Solution:
$$\frac{100.0\text{g}}{18.0\text{g}\;\text{mol}^{-1}}\;=\;5.56\text{mol of}\;H_2O$$
Add that to the 0.100 mol of NaCl = 5.56 + 0.100 = 5.66 mol total
$$\text{Mole fraction of NaCl}\;=$$
$$\frac{0.100\text{mol}}{5.66\text{mol}}\;=\;0.018$$
What is the mole fraction of the H2O?
$$\frac{5.56\text{mol}}{5.66\text{mol}}\;=\;0.982$$

