How to use Percentage Yield Calculator:
According to the formula, it requires two values, first is the Theoretical Yield and the second is Actual Yield.
Example :
$$2\text{AL}\;+\;6\text{HCL}\;\rightarrow\;2\text{ALCL}_3\;+\;3\text{H}_2$$
95 g of hydrochloric acid are reacted with excess aluminum producing 2.2 g of hydrogen gas.What is percentage yield?
Solution :
Calculate theoretical yield :
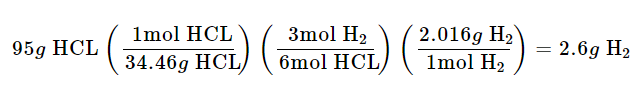
Next, Calculate % Yield :
$$\text{Percentage Yield}\;=$$
$$\frac{\text{Actual}}{\text{Theoretical}}\;*\;100$$
$$\text{Percentage Yield}\;=$$
$$\frac{2.2g\;\text{H}_2}{2.6g\;\text{H}_2}\;*\;100$$
$$\text{Percentage Yield}\;=\;8.6 % $$

